Directory

Saiju Jacob
Consultant NeurologistDr Saiju Jacob is a Consultant Neurologist at University Hospitals of Birmingham and an Honorary Reader at the University of Birmingham. He began his neurology training at Atkinson Morley’s/St George’s Hospital in London before completing his specialist training in the West Midlands.
He undertook a fellowship in neuromuscular and neuroimmunology at the University of Oxford, working under Prof. Angela Vincent and Dr David Hilton-Jones. His doctoral research focused on myasthenia gravis, exploring its clinical, immunological, and neurophysiological aspects.
Dr Jacob specialises in neuromuscular disorders, neuroimmunology, myasthenia gravis, autoimmune encephalitis, and general neurology. His work combines patient care with cutting-edge research, particularly on neuroimmunological conditions affecting the peripheral and central nervous systems.
view this profile

Girish Vajramani
Consultant NeurosurgeonMr Girish Vajramani, MCh (Neuro), DNB (Neuro), FRCS, FRCS (NeuroSurg Glasgow) has been a Consultant Neurosurgeon since 2010, a leading expert in spinal disorders and neurovascular conditions. He has performed numerous spinal surgeries, including transplant surgery for spinal reconstruction. Renowned for treating trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia, he introduced balloon compression treatment for trigeminal neuralgia at Southampton and employs microvascular decompression and PENS therapy for complex facial pain.
At the Wessex Neurological Centre, he has established a functional neurosurgery practice, leading epilepsy surgery, spinal cord stimulation, and deep brain stimulation programmes. He also oversees chronic pain management and intrathecal baclofen therapy.
Trained at NIMHANS in Bangalore, he advanced his expertise in Cardiff and Southampton before completing a functional neurosurgery fellowship at the Walton Centre in Liverpool.
view this profile

Dr. Syamantak Srivastava - Neurosurgeon in Lucknow
Dr. Syamantak Srivastava - Neurosurgeon in LucknowDr. Syamantak Srivastava, MBBS, MS, MCh (Neurosurgery), is a distinguished Brain, Spine, and Peripheral Nerve Surgeon, acclaimed as the best neurosurgeon in Lucknow. With extensive training and experience, he excels in diagnosing and treating complex neurological conditions. Dr. Srivastava is renowned for his expertise in brain and spine surgeries, employing cutting-edge techniques and advanced technology to ensure optimal outcomes. His compassionate approach, combined with a commitment to patient-centric care, has earned him the trust and respect of his patients. Dedicated to continuous learning, Dr. Srivastava stays abreast of the latest advancements in neurosurgery, providing world-class care in Lucknow.
Phone number:
+91 8858595454
Business Hours:
Mon - Sat:10:00 AM - 07:00 PM
Sun :10:00 AM - 02:00 PM
Contact Email ID:
neurodentica@gmail.com
view this profile
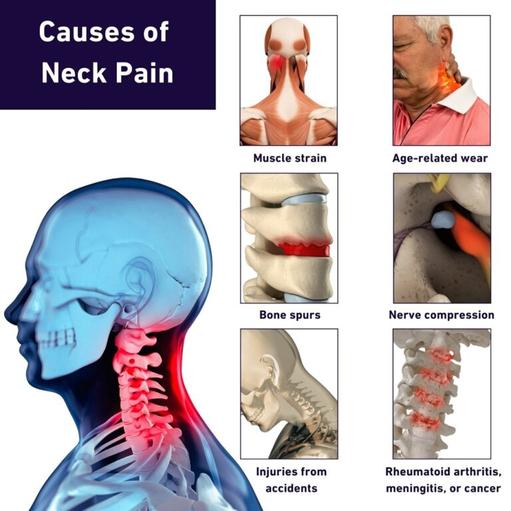
Neck pain is a common complaint affecting many people worldwide. It can come from simple things like sitting with poor posture for a long time, especially if you spend a lot of time on the computer or doing work that strains your neck muscles. Occasionally pain in neck muscles and nerves can be more serious, especially if it’s linked to osteoarthritis or another degenerative disease. While neck pain might seem to some to be a minor complain, it can sometimes be a sign of more serious problems. For that reason, it’s important to seek medical advice if you are suffering from unexplained neck pain. In particular, if you experience symptoms like numbness or weakness in your arms or hands, or if the pain spreads into your shoulder or down your arm, it’s important to see a doctor right away.
What is Neck Pain?
Neck pain appears in many different ways, causing various problems and discomfort that can make it hard to do everyday things. People who suffer with neck pain often feel it getting worse when they keep their head still for a long time, like when they are driving for an extended period or sitting at a desk working on the computer without a lot of movement.
Tight muscles and spasms in the neck can be caused by this lack of movement, but it can also be the result of straining the neck. This might happen through physical work requiring you to hold your head at a slightly awkward angle or through living heavy weights. These situations can make the discomfort worse, making it harder to move your head and sometimes causing headaches.
Although these symptoms may not seem serious, they can have a serious impact on your ability to function and carry out your normal daily activities. To maintain the pain-free mobility of your head, it’s important to take action to manage and treat neck pain early on.
Princeton Brain, Spine and Sports Medicine
731 Alexander Rd #200,
Princeton, NJ 08540
Office tel: (609) 921-9001
Fax: (215) 741-3143
Web address: https://www.princetonbrainandspine.com/
Office location: https://www.princetonbrainandspine.com/locations/nj/princeton/
Our location on map: https://maps.app.goo.gl/eeozrju3JjgJUGmo8
https://plus.codes/87G789C7+W4
Nearby Locations:
Princeton Junction, Plainsboro Center, Princeton North, Lawrenceville, Clarksville
08536, 08540, 08550, 08648
Working Hours: Mon-Fri: 9am-5:00pm
Payment: cash, check, credit cards.

Sciatica is a common condition that causes pain, numbness, and tingling in the lower back, buttocks, and legs. The sciatic nerve runs from the lower back down through the hips and buttocks and into each leg. When this nerve becomes compressed or irritated, the resulting pain is known as sciatica. It is characterized by a sharp, shooting pain that radiates down one or both legs, making everyday activities like walking or sitting difficult.
What is Sciatica?
The sciatic nerve plays a crucial role in providing feeling and movement to the lower body. However, when it’s affected by problems like a herniated disc, arthritis, or excess bone growth, it can lead to pain and inconvenience, the hallmark symptoms of sciatica.
Symptoms of Sciatica
Sciatica often begins in the lumbar spine due to natural degenerative processes and can cause a range of painful symptoms, including:
- Discomfort typically starts in the lower back and can travel down to the buttocks, thigh, and calf.
- Sciatica pain intensity can vary from a dull throb to a sharp, shooting sensation.
- Sometimes, it feels like an electric shock down the leg.
- Certain actions like coughing, sneezing, or prolonged sitting can worsen symptoms.
- Sciatica usually affects one side of the body, causing asymmetrical symptoms.
- Sciatica brings not just pain but also sensory issues that add to the distress of the person suffering.
This might include feelings of numbness, tingling, or weakness in the affected limb, making it harder to use normally. It can be a strange contrast: while one part of the leg is in intense pain, another part feels oddly numb. This mix of sensations highlights the particular characteristic of sciatica, blending different feelings to contribute to your discomfort.
Read more: https://www.princetonbrainandspine.com/conditions/spinal/sciatica/
Princeton Brain, Spine and Sports Medicine
731 Alexander Rd #200,
Princeton, NJ 08540
Office tel: (609) 921-9001
Fax: (215) 741-3143
Web address: https://www.princetonbrainandspine.com/
Office location: https://www.princetonbrainandspine.com/locations/nj/princeton/
Our location on map: https://maps.app.goo.gl/eeozrju3JjgJUGmo8
https://plus.codes/87G789C7+W4
Nearby Locations:
Princeton Junction, Plainsboro Center, Princeton North, Lawrenceville, Clarksville
08536, 08540, 08550, 08648
Working Hours: Mon-Fri: 9am-5:00pm
Payment: cash, check, credit cards.
Directory:
Tags:

Artificial disc replacement, or disc arthroplasty, is an innovative surgical procedure to restore stability in the spinal column when cushioning discs between vertebrae have been damaged or deteriorated. Princeton Brain, Spine & Sports Medicine’s experienced neurosurgeons are fellowship-trained to perform this state-of-the-art procedure at leading affiliated hospitals in PA and NJ.
What Is Artificial Disc Replacement?
For years, the traditional treatment for herniated, damaged or degenerated spinal discs has been a procedure called spinal fusion, where two or more vertebrae are joined together to stabilize the spine and alleviate pain in the neck and back. This fusion of vertebrae results in a loss of spinal flexibility. With artificial disc replacement, the damaged disc is restored with an FDA-approved replacement disc consisting of two metal plates or two metal plates separated by plastic padding. The metal plates glide over one another and the plastic disc, restoring the natural function of the spine and preserving the patient’s ability to bend forward, backward and side-to-side.
Princeton Brain, Spine and Sports Medicine
731 Alexander Rd #200,
Princeton, NJ 08540
Office tel: (609) 921-9001
Fax: (215) 741-3143
Web address: https://www.princetonbrainandspine.com/
Office location: https://www.princetonbrainandspine.com/locations/nj/princeton/
Our location on map: https://maps.app.goo.gl/eeozrju3JjgJUGmo8
https://plus.codes/87G789C7+W4
Nearby Locations:
Princeton Junction, Plainsboro Center, Princeton North, Lawrenceville, Clarksville
08536, 08540, 08550, 08648
Working Hours: Mon-Fri: 9am-5:00pm
Payment: cash, check, credit cards.

Princeton Brain, Spine and Sports Medicine
Dr. Mark Robert Mclaughlin, MDAward-Winning Neurosurgery & Spine Care in New Jersey and Eastern Pennsylvania. Experience life-changing relief from brain, spine, and joint injuries with our best orthopedic spine surgeonstop neurosurgeons and sports medicine specialists. Customized to your unique needs, our expert team will guide you towards a rapid recovery and a pain-free life.
Proudly serving patients in NJ and PA for nearly 20 years, our practices are home to award-winning, patient-focused doctors. Beginning with a precise diagnosis, we focus on educating patients at every stage of treatment, providing the industry’s most advanced treatment technology, and a range of conservative and surgical options.
Our multi-disciplinary team of board-certified doctors has undergone extensive training in their respective areas, providing highly specialized treatments and customized therapies delivered with compassion.
Princeton Brain, Spine, & Sports Medicine accepts most major insurance plans. Call us today to schedule a consultation or learn more about our outstanding practice.
At our spine surgery and neurosurgery center, you can make use of the following procedures:
- Spinal surgery
- back pain treatment
- neck pain treatment near me
- sciatica treatment
- Herniated disc treatment
- Anterior lumbar fusion
Princeton Brain, Spine and Sports Medicine
731 Alexander Rd #200,
Princeton, NJ 08540
Office tel: (609) 921-9001
Fax: (215) 741-3143
Web address: https://www.princetonbrainandspine.com/
Office location: https://www.princetonbrainandspine.com/locations/nj/princeton/
Our location on map: https://maps.app.goo.gl/eeozrju3JjgJUGmo8
https://plus.codes/87G789C7+W4
Nearby Locations:
Princeton Junction, Plainsboro Center, Princeton North, Lawrenceville, Clarksville
08536, 08540, 08550, 08648
Working Hours: Mon-Fri: 9am-5:00pm
Payment: cash, check, credit cards.
Our social links:
https://www.facebook.com/BrainSpineMD/
https://www.linkedin.com/in/mmclaughlinmd
https://www.linkedin.com/company/princeton-brain-&-spine-care/
https://www.instagram.com/brainspinesportsmed/
https://www.youtube.com/@brainspinemd726
https://www.pinterest.com/princetonbrainandspine/
https://www.yelp.com/biz/princeton-brain-and-spine-princeton-2
https://www.tiktok.com/@princetonbrainspine
Find us at: https://www.doximity.com/pub/mark-mclaughlin-md
View other locations Princeton Brain, Spine and Sports Medicine has been mentioned
https://birdeye.com/princeton-brain-spine-and-sports-medicine-157868801602816
https://www.bbb.org/us/nj/princeton/profile/neurosurgeons/princeton-brain-spine-0221-90191346
view this profile
Directory:
Tags:

Proudly serving patients in NJ and PA for nearly 20 years, our practices are home to award-winning, patient-focused doctors. Beginning with a precise diagnosis, we focus on educating patients at every stage of treatment, providing the industry’s most advanced treatment technology, and a range of conservative and surgical options.
Our multi-disciplinary team of board-certified doctors has undergone extensive training in their respective areas, providing highly specialized treatments and customized therapies delivered with compassion.
Princeton Brain, Spine, & Sports Medicine accepts most major insurance plans. Call us today to schedule a consultation or learn more about our outstanding practice.
Princeton Brain, Spine and Sports Medicine
731 Alexander Rd #200,
Princeton, NJ 08540
Office tel: (609) 921-9001
Fax: (215) 741-3143
Web address: https://www.princetonbrainandspine.com/
Office location: https://www.princetonbrainandspine.com/locations/nj/princeton/
Our location on map: https://maps.app.goo.gl/eeozrju3JjgJUGmo8
https://plus.codes/87G789C7+W4
Nearby Locations:
Princeton Junction, Plainsboro Center, Princeton North, Lawrenceville, Clarksville
08536, 08540, 08550, 08648
Working Hours: Mon-Fri: 9am-5:00pm
Payment: cash, check, credit cards.


Dr. Mark Robert Mclaughlin, MD
NeurosurgeonTo me, it was never a question of whether I would become a doctor but what area of medicine I’d choose. My grandfather, my idol, was a doctor in West Orange, New Jersey, for more than 50 years. As a kid, I followed him around constantly, and sometimes even carried his medical bag on house calls.
But when I entered college, I opted to major in philosophy, rather than follow the conventional pre-med track. My interests spanned beyond medicine. And, ultimately, I thought if I could think, speak, and write clearly, I’d be a better doctor.
While I’ve always considered myself an avid student, and still do, much of what I’ve come to understand about life I’ve learned on the wrestling mat. I was a Division I wrestler in college, and one of my proudest achievements was being inducted into the National Wrestling Hall of Fame in 2016.
For the better part of the last 20 years, I’ve coached 8- to 12-year-olds with the Princeton Wrestling Club in the town where I live. And five years ago, I helped launch Trenton Youth Wrestling, a program that each year enables 200 girls and boys from the city’s public schools to learn about and compete in the sport.
I’ve remained involved in wrestling because I believe its principles can help shape the trajectory of a child’s life. That’s what happened to me. I credit wrestling and an inspiring coach early in my life with giving me the mental and physical tools to do my best, on the mat and in the operating room. Wrestling taught me the importance of perseverance and gave me the fortitude to achieve a successful career, from pursuing a challenging residency under Dr. Peter Jannetta, the father of modern neurosurgery, to surrounding myself with some of the brightest minds working in the field today.
In recent years, I’ve settled into a slightly less prominent role within our practice so that I can devote more time to my patients. I continue to focus on cervical spine surgery, which has been revolutionized by the M6-C artificial cervical disc replacement. Designed to restore the natural range of movement to the spine, the M6-C disc is intended as an alternative to cervical fusion. Patients once crippled by pain who’ve undergone the procedure are consistently regaining a sense of normalcy in their lives.
My practice welcomes referrals for most brain and spine disorders, and we accept most major insurance providers, as well as Medicare.
Princeton Brain, Spine and Sports Medicine
731 Alexander Rd #200,
Princeton, NJ 08540
Office tel: (609) 921-9001
Fax: (215) 741-3143
Web address: https://www.princetonbrainandspine.com/
Office location: https://www.princetonbrainandspine.com/locations/nj/princeton/
Our location on map:
https://maps.app.goo.gl/eeozrju3JjgJUGmo8
https://plus.codes/87G789C7+W4
Nearby Locations:
Princeton Junction, Plainsboro Center, Princeton North, Lawrenceville, Clarksville
08536, 08540, 08550, 08648
Working Hours :
Mon-Fri: 9am-5:00pm
Payment: cash, check, credit cards.
view this profile




