- Each year about 1.7m women are diagnosed with breast cancer worldwide and over 0.5m die from the condition
- Between 5% and 10% of these breast cancers result from harmful gene mutations
- BRCA1 and BRCA2 gene mutations are the most common cause of hereditary breast cancer
- 45% to 85% of women with a BRCA mutation will develop breast cancer in their lifetime compared to 12% of women in the general population
- Most women do not know if they have a harmful BRCA mutation
- Testing for the BRCA gene is now affordable, fast and accessible
- Surgical interventions of women with BRCA mutations can significantly reduce their risk of developing breast cancer and substantially increase cancer survival
- Genetic test results for breast cancer are fraught with uncertainty because testing reveals the likelihood of developing cancer rather than a certain fate
- Research suggests that BRCA test results are not being clearly communicated to women
- Best practice demands that expert counselors discuss genetic testing and help interpret results
Breast cancer and harmful BRCA gene mutations
Few things frighten women more than discovering a lump in one of her breasts The standard treatment: surgery, followed by radio- and chemotherapy, can be disfiguring, painful, sometimes unsuccessful, and the impact of the disease is felt by far more individuals than just those who have the diagnosis.The good news is that over the past 30 years breast cancer survival rates in most developed countries have been improving, largely due to screening, earlier diagnosis and improved treatments. The bad new is that in most developed countries it is twice as likely for a woman to be diagnosed with breast cancer than 60 years ago.
Harmful BRCA genes mutations
5 to 10% of breast cancers are thought to be due to gene mutations, and harmful BRCA mutations account for 20 to 25% of these. Women who inherit the BRCA1 mutations have a 60 to 90% risk of developing breast cancer in their lifetime, and those who inherit BRCA2 mutations increase their risk of breast cancer by 45 to 85%, compared to 12% of women in the general population. Most women do not know if they carry the harmful BRCA mutation, but if they discover they do, many elect to have a bilateral mastectomy. This is a significant procedure with potential risks and side effects, but can reduce your mortality risk by about 50%.
The gold standard screening for breast cancer is an x-ray picture of the breast (mammography), but increasingly women are turning to genetic testing as their awareness of the harmful BRCA mutations increase, and genetic testing becomes more accessible and affordable. However, results from these tests are not straightforward, and often not communicated well. This can increase the anxiety in women with suspected breast cancer, and make them elect to have unnecessary interventions and procedures.
This Commentary describes how advanced genetic testing together with expert counselling help women improve their management of breast cancer.
Breast Cancer
Cancer is a group of diseases that cause cells in your body to change and grow out of control: they mutate. Most types of cancer cells eventually form a lump or mass called a tumor, and are named after the part of the body where the tumor originates, e.g. “breast cancer”, although this convention is changing with the development of targeted personalized medicine. The exact cause of breast cancer is unknown, but the overwhelming majority result from some combination of environment, lifestyle, and genes. Breast cancer affects about 1 in 8 women at some point during their life, usually after the menopause, and is the most common cancer in women. The majority of breast cancers begin in the parts of the breast tissue that are made up of glands for milk production, called lobules, and ducts that connect the lobules to the nipple. The remainder of the breast is made up of fatty, connective, and lymphatic tissue. Most invasive breast cancers (those that have spread from where they started) are found in women 55 and older. Women with a family history of the disease have an increased risk of getting breast cancer. Each year about 1.7m women are diagnosed with breast cancer worldwide, and over 0.5m die from the condition. However in developed economies more and more women survive the disease. In the US, for instance, the average 5-year survival rate for people with breast cancer is 89%. The 10-year rate is 83%, and the 15-year rate is 78%. Other developed countries have similar success rates. What makes breast cancer fatal is if it spreads to the bones, lungs, liver and other organs. Early detection in order to improve breast cancer outcomes remains the cornerstone of the condition’s management. Although breast cancer is thought to be a disease of the developed world, it is increasing rapidly in emerging countries where the majority of cases present later and die earlier than women in developed countries: almost 50% of breast cancer cases and 58% of deaths occur in emerging economies. This is because women generally have relatively poor knowledge of the risk factors, symptoms and methods for early detection. Also, they experience cancer fatalism, believe in alternative medicine, and lack of autonomy in decision making, which often results in delays in seeking or avoidance of evidence-based medicine.
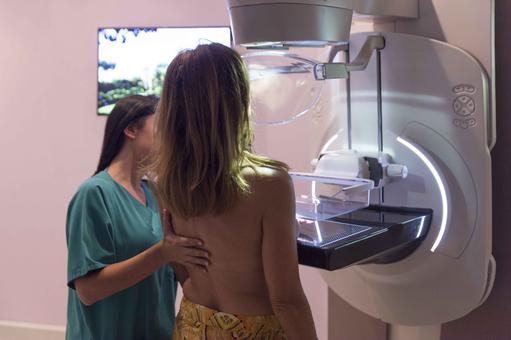
Mammography
Mammography, which has long been the mainstay of breast cancer detection, is a specific type of breast imaging that uses low-dose x-rays to detect small changes in the breast before there are any other signs or symptoms of the disease when it is most treatable. Mammography is noninvasive, relatively inexpensive, and has reasonable sensitivity (72–88%), which increases with age. It can also be used to detect and diagnose breast disease in women experiencing symptoms such as a lump, pain, or nipple discharge. If breast cancer is found at an early stage, there is an increased chance for breast-conserving surgery and a better prognosis for long-term survival. Most developed countries operate breast-screening programs, which regularly provides mammography for women between certain ages.
Advances in mammography
In recent years, mammography has undergone increased scrutiny for false positives and excessive biopsies, which increase radiation dosage, cost and patient anxiety. In response to these challenges, new forms of mammography screening have been developed, including; low dose mammography, digital mammography, computer-aided detection, tomosynthesis, which is also called 3-D mammography, automated whole breast ultrasound, molecular imaging and MRI. Notwithstanding, there is increasing awareness of subpopulations of women for whom mammography has reduced sensitivity. More recently, women have turned to genetic testing to gain a better understanding of their risk of inherited breast cancer.
Genes
Every cell in your body contains genes. These contain the genetic code for your body, which not only determines the color of your eyes and hair etc., but also provides information that affects how the cells in your body behave: for example, how they grow, divide and die. Information in your genes is inherited from both parents, and you pass on this information to your children. A change in your genetic code that affects the function of a gene is called a mutation. Many inherited gene mutations do not have any effect on your health, but some do; the BRCA1 and BRCA2 mutations account for 20 to 25% of all inheritable female breast cancers and 15% of ovarian cancers.
BRCA genes
In normal cells, BRCA genes are tumor suppressor genes that assist in preventing cancer developing by making proteins that help to keep cells from growing abnormally. Mutated versions of BRCA genes cannot stop abnormal growth, and this can lead to cancer. Mutated BRCA genes have a higher prevalence in certain ethnic groups, such as those of Ashkenazi Jewish descent.
In the video below Professor Robert Leonard, a medical oncologist and an authority on breast cancer, describes how BRCA genes are influential in breast and ovarian cancer risk. BRCA1 runs in families and may also increase a woman’s risk of developing fallopian tube and peritoneal cancers. BRCA2 also runs in families, and is more breast cancer-specific, but a less commonly inherited abnormality. Both or either of these genes may not be detectably abnormal even in a family with a strong inherited pattern of breast cancer, but there is a significant possibility that you will find them in people with a family history of breast and ovarian cancer. Breast and ovarian cancers associated with BRCA mutations tend to develop at younger ages than their non-hereditary counterparts.
 Enhanced risk when family members have cancer
In December 2013, the US Preventive Services Task Force recommended that women who have family members with breast, ovarian, fallopian tube, or peritoneal cancer be evaluated to see if they have a familial history that is associated with an increased risk of a harmful mutation in one of the BRCA genes. Compared to women without a family history of cancer, risk of breast cancer is about 2 times higher for women with a close female relative who has been diagnosed with cancer; nearly 3 times higher for women with two relatives, and nearly 4 times higher for women with three or more relatives. Risk is further increased when the affected relative was diagnosed at a young age. Notwithstanding, the Preventive Services Task Force recommends against BRCA testing for women with no family history of cancer.
The Angelina Jolie effect
The Hollywood actress and filmmaker Angelina Jolie lost her grandmother and aunt to breast cancer and her mother to ovarian cancer. After discovering that she carried a maternally inherited pathogenic BRCA1 mutation, and being told that she had an 87% chance of developing breast cancer, and a 50% chance of ovarian cancer, Jolie elected to have her breasts, ovaries and fallopian tubes removed. After surgery her risk of developing breast cancer in later life fell to 5%.
In May 2013, Jolie described her decision in a New York Times (NYT) article, “I am writing about it now because I hope that other women can benefit from my experience . . . . . Cancer is still a word that strikes fear into people’s hearts, producing a deep sense of powerlessness. But today it is possible to find out through a blood test whether you are highly susceptible to breast and ovarian cancer, and then take action.”
Over testing of by low-risk women
Findings published in December 2016 in the British Medical Journal suggest that tests for the BRCA genes shot up by 64% following Jolie’s article. Researchers analysed data on US health insurance claims from more than 9m women between 18 and 64, and suggested that in just 2 weeks following Jolie’s NYT disclosure, 4,500 additional BRCA tests were carried out, which cost the US healthcare system some US$13.5m. Interestingly, increased testing rates were not accompanied by a corresponding increase in mastectomy rates, which suggests that additional testing did not identify new BRCA mutations. Thus, the Angela Jolie effect might have encouraged over-testing among low-risk women.
Mindful of her influence on women’s decisions, in 2015 Jolie wrote another NYT article in which she attempted to correct her earlier support for radical risk reduction surgery for women carriers of BRCA mutations. She said that because surgery worked for her, it is not necessarily the optimal therapeutic pathway for all women, and stressed that non-surgical treatments could be more appropriate.
Traditional genetic testing for breast cancer risk was slow and expensive
Genetic testing to detect BRCA mutations has been available since 1996, but for many years it was under-used because of its scarcity, high cost, and the length of time it took to produce a result. The rapid development and plummeting costs of genetic testing, and a 2013 US Supreme Court ruling, which invalidated the patents held by Myriad Genetics Inc., which restricted BRCA testing, have resulted in the growth and accessibility of genetic testing.
BRCA testing is not straightforward
There are hundreds of mutations in the BRCA1 and BRCA2 genes that can cause cancer. Several different tests are available, including tests that look for a known mutation in one of the genes (i.e., a mutation that has already been identified in another family member), and tests that check for all possible mutations in both genes. Commercial laboratories usually charge between US$450 and US$5,000 to carry out BRCA testing, depending on whether you are being tested for only a specific area(s) of a gene known to be abnormal or if hundreds of areas are being examined within multiple genes. Tests that use traditional technology take several months to report findings. This means that even if a woman is tested at the time of diagnosis, she might not know the results before she has to decide on treatment.
Importance of regulated testing laboratories
Testing for the BRCA genes usually involves a blood sample taken in a doctor’s clinic and sent to a commercial laboratory. In 1988, the US Congress passed the Clinical Laboratory Improvement Amendments (CLIA) to ensure quality standards, and the accuracy and reliability of results across all testing laboratories. Since then, all legitimate genetic testing in the US is undertaken in CLIA-approved facilities. During testing for BRCA mutations, the genes are separated from the rest of the DNA, and then scanned for abnormalities. Unlike other clinical screening such as HIV tests and colonoscopies, which provide a simple positive or negative result; genetic testing is fraught with uncertainty because it reveals the likelihood of developing cancer rather than a certain fate.
BRCA1 and BRCA2 genetic test results
A positive BRCA test result indicates that you have inherited a known harmful mutation in the BRCA1 or BRCA2 gene. This means that you have an increased risk of developing breast and ovarian cancers, but it does not mean that you will actually develop cancer. Some women who inherit a harmful BRCA mutation will never develop cancer. A positive test result may create anxiety and compel clinicians to perform further tests and women to undergo premature and unnecessary clinical interventions, other women in a similar situation will opt for regular screening.
The potential benefits of a true negative result include a sense of relief regarding your future risk of cancer, learning that your children are not at risk of inheriting the family's cancer susceptibility, and that a range of interventions may not be required. However, a negative result sometimes can be difficult to interpret because its meaning partly depends on your family’s history of cancer, and whether a BRCA mutation has been identified in a blood relative. Further, scientists continue to discover new BRCA1 and BRCA2 mutations, and have not yet identified all potentially harmful ones. Therefore, it is possible that although you have a “negative” test result you might have a harmful BRCA1 or BRCA2 mutation, which has not been identified.
Counselling
Because of these uncertainties and the agonising choices women with suspected breast cancer face, health providers in most developed countries recommend counselling as part of breast cancer treatment pathways. In the video below Dr John Green, a medical oncologist knowledgeable about the influence of inherited BRCA gene mutations on treatment options underlines the importance of expert genetic counselling to help women navigate their therapeutic pathways. Counselling is performed by a health professional experienced in cancer genetics, and usually includes the psychological risks and benefits of genetic tests, a hereditary cancer risk assessment based on a person’s personal and family medical history; a description of the tests, their technical accuracy and appropriateness, medical implications of a positive or a negative test result, the possibility of uncertain or ambiguous test results, cancer risk-reducing treatment options, and the risk of passing on a mutation to children. Because people are more aware of the genetic mutations linked to breast cancer, the demand for genetic testing and counselling have increased, and in some instances it is challenging for genetic counsellors to keep pace with demand.

The context in which genetic tests are carried out
A 2017 study published in the Journal of Clinical Oncology suggests that genetic test results for breast cancer are not being clearly communicated to women, and this could cause them to opt for treatments that are more aggressive than they actually need. To reduce this possibility the Royal Marsden NHS Trust Hospital in London has introduced the Mainstreaming Cancer Genetics programme. Since 2014 the Marsden has employed genetic counseling and used laboratories with enhanced genetic testing capabilities. This reduces processing time and costs, helps to meet the increased demand for rapid, accurate and affordable BRCA testing, and helps women make critical decisions about their treatment options.
“There were two main problems with the traditional system for gene testing. Firstly, gene testing was slow and expensive, and secondly the process for accessing gene testing was slow and complex,” says Nazneen Rahman, Professor and Head of Cancer Genetics at the UK’s Institute for Cancer Research in London. “We used new DNA sequencing technology to make a fast, accurate, affordable cancer gene test, which is now used across the UK. We then simplified test eligibility and brought testing to patients in the cancer clinic, rather than making them have another appointment, often in another hospital,” says Rahman.
The Marsden is now offering tests to three times more patients a year than before the program started. The new pathway is faster, with results arriving within 4 weeks, as opposed to the previous 20-week waiting period. According to Rahman, “Many other centres across the country and internationally are adopting our mainstream gene testing approach. This will help many women with cancer and will prevent cancers in their relatives.”
Takeaways
The history of cancer is punctuated with overzealous interventions, many of which have had to be modified once it has been demonstrated that they could cause more harm than good.
As advanced genetic testing becomes affordable and more accessible it is important that their results are interpreted with the help of genetic counsellors in a broader familial context in order to help women make painfully difficult decisions about their treatment.
Migration to next generation genetic testing technologies has many benefits, but it also introduces challenges, which arise from, the choice of platform and software, and the need for enhanced bio-informatics analysts, which are in scarce supply. An efficient, cost-effective accurate mutation detection strategy and a standardized, systematic approach to the reporting of BRCA test results are central for diagnostic laboratories wishing to provide a service during a time of increasing demand and downward pressure on costs.
|