Tag
Tagged: diabetes management
Directory:
Tags:
- A 2017 research project found that only 6 out of 18 FDA-approved blood glucose monitoring (BGM) systems tested were accurate
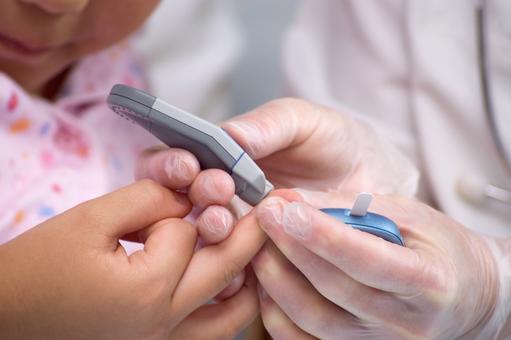
- Each day BGM systems are used by millions of people with diabetes to help them self-manage their condition, and avoid devastating and costly complications
- Thousands of similar smart devices support the prevention and self management of other chronic lifetime conditions, whose prevalence levels are high
- The increasing demand for healthcare, its escalating costs, and rapidly evolving technologies are driving the growth of such remote self-managed devices
- The most valuable aspect of such devices is the data they produce
- These data tend to be under valued and under utilized by healthcare providers
- This has created an opportunity for giant technology companies to enter the healthcare market with a plethora of smart devices and start utilizing the data they collect to enhance patient outcomes and lower costs
- Giant technology companies could dis-intermediate GPs and re-engineer primary care
Digital blood glucose monitors and the disruptive impact of giant tech companies on healthcare
A 2017 research project, which tested 18 FDA-approved digital blood glucose monitoring (BGM) systems, which are used daily by millions of people with diabetes to check the concentration of glucose in their blood, found that only 6 were accurate. The research, led by David Klonoff of the Diabetes Research Institute at San Mateo, California, was funded by Abbott Laboratories.
This Commentary describes both traditional and next-generation BGM systems, and Klonoff’s research. The Commentary suggests that BGM systems are just one part of a vast, global, rapidly growing market for consumer healthcare devices, and argues that the most valuable aspect of these devices is the data they collect. With some notable exceptions, healthcare professionals do not optimally utilize these data to enhance care and reduce costs. This has created for an opportunity for technology companies to enter the healthcare market and re-engineer primary care. The one thing, which might slow the march of giant technology companies into mainstream healthcare, is the privacy issue.
Traditional and next-generation BGM systems
Traditional BGM systems
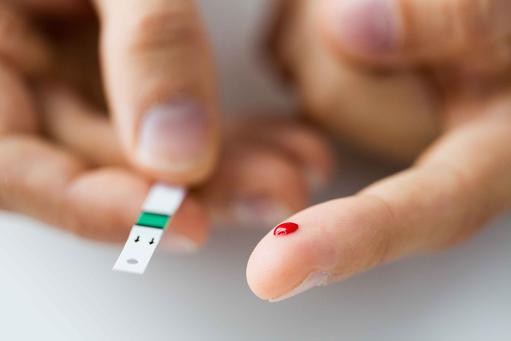
Regularly, each day, BGM systems are used by millions of people with diabetes to help them manage their condition. Managing diabetes varies from individual to individual, and peoples with diabetes usually self-monitor their blood glucose concentration from a small drop of capillary blood taken from a finger prick. They then apply the blood to a chemically active disposable 'test-strip'. Different manufacturers use different technology, but most systems measure an electrical characteristic, and use this to determine the glucose level in the blood. Such monitoring is the most common way for a person with diabetes to understand how different foods, medications, and activities affect their condition. The challenge for individuals with diabetes is that blood glucose levels have to be tested up to 12 times a day. People obliged to do this find finger pricking painful, inconvenient and intrusive, and, as a consequence, many people with diabetes do not check their glucose levels as frequently as they should, and this can have significant health implications. If your levels drop too low, you face the threat of hypoglycemia, which can cause confusion or disorientation, and in its most severe forms, loss of consciousness, coma or even death. Conversely, if your blood glucose levels are too high over a long period, you risk heart disease, blindness, renal failure and lower limb amputation.Next generation BGM system
Abbott Laboratories Inc. markets a BGM system, which eliminates the need for routine finger pricks that are necessary when using traditional glucose monitors. Instead of finger pricks and strips, the BGM system, which measures interstitial fluid glucose levels, comprises a small sensor and a reader. An optional companion app for Android mobile devices is also available. The sensor is a few centimetres in diameter and is designed to stay in place for 10 days. It is applied to the skin, usually on the upper arm. A thin (0.4 mm), flexible and sterile fibre within the sensor is inserted in the skin to a depth of 5 mm. The fibre draws interstitial fluid from the muscle into the sensor, where glucose levels are automatically measured every minute and stored at 15-minute intervals for 8 hours. Glucose levels can be seen at any time by scanning the reader over the sensor. When scanned the sensor provides an answer immediately. It also shows an 8-hour history of your blood glucose levels, and a trend arrow showing the direction your glucose is heading. The device avoids the pain, and inconvenience caused by finger-prick sampling, which can deter people with diabetes from taking regular measurements. In the UK the system costs £58 for the reader, plus £58 for a disposable sensor, which must be replaced every 10 days and from November 2017 have been available on the NHS. Abbott Laboratories is a global NASDAQ traded US MedTech Company, with a market cap of US$86bn; annual revenues of US$21bn, and a diabetes care division, which produces annual revenues of some US$600m.
Klonoff’s research on BGM systems
BGM systems used by Klonoff and his team for their research were acquired over-the-counter and independent of their manufacturers. All were tested according to a protocol developed by a panel of experts in BGM surveillance testing.
Klonoff’s research specified that for a BGM system to be compliant, a blood glucose value must be within 15% of a reference plasma value for a blood glucose >100 mg/dl, and within 15 mg/dl of a reference plasma value for a blood glucose approved” a BGM system had to pass all 3 trials. Only 6 out of 18 passed by achieving an overall compliance rate of 95% or higher.
|
|
Directory:
Tags:
|









